Rebecca Shindel
Executive Director, Business Analytics, MIRADOR Global
Forecasting a pharmaceutical product’s launch and lifecycle sales can be challenging. It requires an understanding of the patients to be treated and the market into which the product will be launched. The forecast must address the expectations of various internal and external stakeholders. It is also an opportunity to drive strategic discussions within an organization. With the right knowledge and collaboration, the product forecast becomes a strategic tool.
Developing a forecast that is also a strategic too enables you to:
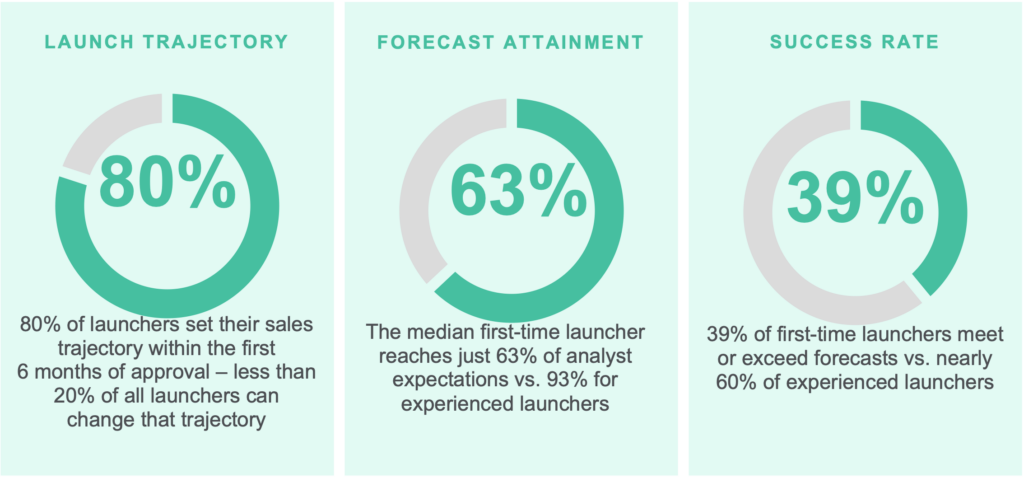
The art of forecasting involves a deep understanding of the patient population based on literature, available data, and any patient journey work. Identifying which patients are eligible for a therapy and where a product fits in the treatment paradigm (including pathways, guidelines, and clinical practice) are critical to accuracy. One study found that only 39% of biotech companies meet or exceed their launch forecast. It may be due to an overstatement of the eligible patients available.
Increasingly, emerging biopharma companies are embarking on the forecasting journey themselves, but with subpar results
In addition to overstating the number of available patients, other pitfalls include:
A robust forecast includes assumptions related to these areas.
A Good Forecast Reflects Thinking Across the Organization
A good forecast will reflect cross-functional viewpoints. When a company develops a forecast, part of the process should be to bring various internal stakeholders together to discuss product potential. These areas include the Executive, Finance, Commercial, Market Access, Operations, Regulatory, and Medical teams. This dialog leads to a set of assumptions that are then built into a patient funnel, which is designed to:
Understanding Stakeholder Expectations
At a recent BIO conference, potential investors indicated that they are looking for three things when evaluating an investment:
A well-supported, robust forecast will seek to address these three questions.
Does the product address an unmet need?
By identifying the appropriate patient population, it can be determined whether there are sufficient patients with unmet need in a particular disease area. Most companies can identify the total number of patients afflicted with a given disease but have a difficult time understanding the relevant subpopulation(s). These can be biomarker-based, line of therapy based, and age- and/or gender-based, among other factors. Some ways to uncover this are:
All three methods provide value but sometimes data costs make claims data analysis undesirable. Many clients are surprised to learn that, depending on where a product is in its development cycle, a literature search can be adequate. At Mirador Global, we suggest that in earlier phases of development (pre-Phase 3), an epi-based forecast using literature as the basis for assumptions will be well accepted.
What is the product’s potential?
With an aligned patient funnel and an understanding of what price is appropriate, a well-documented forecast can be developed. There are several ways to determine an appropriate price:
A coordinated approach using both methods and then synthesizing the findings has been successful with our clients.
What is the pathway to reimbursement?
Often, the pathway to reimbursement is uncovered by talking with payer stakeholders and decision makers. A study of the product’s expected competitors’ payer strategies or an analog market can provide robust insight into what reimbursement might look like, especially at launch. Some of the other things to consider are:
By using the forecast as a strategic tool, rather than just an analytical exercise, companies can develop a robust patient funnel, gather insights from across the organization to inform the forecast, and develop the information that will address stakeholder expectations.

We are always available to answer your forecasting questions and welcome the opportunity to brainstorm on the best forecasting approach for your product and its stage of development. Please contact the author of this paper, Rebecca Shindel, at RebeccaShindel@MiradorGlobal.com.
